Biochemistry of muscle, muscle contraction.
Muscle
Muscle (from Latin musculus "little mouse" is contractile tissue of the body and is derived from the mesodermal layer
of embryonic germ cells.
It is classified as:
skeletal, cardiac, or smooth muscle.
Function of muscle is to
produce force and cause motion,
either locomotion or movement within internal organs. Much of muscle contraction occurs without consciousthought
and is necessary for survival, like the contraction of the heart,
or peristalsis (which pushes food through the digestive system).
Voluntary muscle contraction is used to move the body, and can be finely
controlled, like movements of the eye, or gross movements like the quadriceps muscle of the thigh.
Muscular System

There are two broad
types of voluntary muscle fibers, slow twitch and
fast twitch. Slow twitch fibers contract for long
periods of time but with little force while fast twitch fibers
contract quickly and powerfully but fatigue very rapidly.
The muscular system includes three
types of muscles. They are smooth, which are found on the walls of internal
organs, cardiac, which is found only in the heart, and
skeletal muscles, which help strenthen the body and
connect to bones.
There are three types of muscle:

· Skeletal muscle or "voluntary muscle" is anchored by tendons to bone and is used to affect skeletal movement such as locomotion and in maintaining posture. Though this postural control is generally
maintained as a subconscious reflex, the muscles responsible react to conscious
control like non-postural muscles. An average adult male is made up of 40-50%
of skeletal muscle and an average adult female is made up of 30-40%.
Skeletal muscle is a type of striated
muscle, which usually attaches to tendons. Skeletal muscles are used
to create movement,
by applying force to bones and joints viacontraction
. They generally contract voluntarily (via somatic nerve stimulation),
although they can contract involuntarily through reflexes. The whole muscle is wrapped in
a special type of connective tissue, epimysium.

Muscle cells (also
called muscle fibers) are cylindrical, and are multinucleated (in vertebrates and insects). The nuclei of
these muscles are located in the peripheral aspect of the cell, just under the plasma membrane, which vacates the
central part of the muscle fiber for myofibrils. (Conversely, when the
nucleus is located in the center it is considered a pathologic condition known
as centronuclear myopathy.)
Skeletal muscles have one end (the "origin") attached to a bone
closer to the centre of the body's axis and the other end (the
"insertion") is attached across a joint to
another bone further from the body's axis. The bones rotate about the joint and
move relative to one another by contraction of the muscle (lifting of the upper
arm in the case of the origin and insertion described here).

Skeletal muscle
cells are stimulated by acetylcholine, which is released at neuromuscular
junctions by motor neurons. Once the cells are "excited", their sarcoplasmic reticulum will release ionic calcium (Ca2+)
which interacts with the myofibrils to induce muscular contraction (via the
sliding filament mechanism). This process also requires adenosine triphosphate (ATP). The ATP is produced by metabolizing creatine phosphate and glucose (stored as glycogen or
absorbed from blood) within the muscle cells by mitochondria, as well as by metabolizing
fatty acids obtained from the blood and within the cell. Each motor neuron
activates a group of muscle cells, and collectively the neurons and muscle
cells are known as motor units. When more strength is required than can be
obtained from a single motor unit, more units will be stimulated; this is known
as motor unit recruitment. This is spatial summation. If more strength is
required than can be obtained from the current number of motor units, the motor
neurons continue to recruit more motor units. When all the motor units are
recruited, there will be no further increase in contraction strength. To
increase the force of contraction, it is necessary to increase the frequency of
neuronalT firing. This results in tetanic
contraction, which is a smooth contraction. This is temporal summation...
· Smooth muscle or "involuntary muscle" is found within the
walls of organs and structures such as the esophagus, stomach, intestines, bronchi, uterus, urethra, bladder, and blood vessels, and unlike skeletal muscle, smooth muscle is not under
conscious control.
Smooth muscle

Smooth muscle is
a type of non-striated muscle, found within the tunica media layer of large and small arteries and veins, the bladder, uterus, male and female reproductive tracts,gastrointestinal tract
, respiratory tract, the ciliary muscle, and iris of the eye. The glomeruli of the kidneys contain a smooth
muscle-like cell called the mesangial cell. Smooth muscle is fundamentally
different from skeletal muscle and cardiac muscle in terms of structure, function,
excitation-contraction coupling, and mechanism of contraction.
Function
To maintain organ
dimensions against forces, cells are fastened to one another by adherens junctions. As a consequence, cells are
mechanically coupled to one another such that contraction of one cell invokes
some degree of contraction in an adjoining cell. Gap junctions couple adjacent
cells chemically and electrically, facilitating the spread of chemicals (e.g.,
calcium) or action potentials between smooth muscle cells. Smooth muscle may
contract spontaneously (via ionic channel dynamic or Cajal
pacemaker cells) or be induced by a number of physiochemical agents (e.g.,
hormones, drugs, neurotransmitters - particularly from the autonomic nervous system), and also
mechanical stimulation (such as stretch).

Smooth muscles
have been divided into "single unit" and "multi-unit" or
into "phasic" and "tonic" types
based on the characteristics of the contractile patterns and characteristics of
the smooth muscle. Multi-unit smooth muscle lines the large airways to the
lungs and large blood vessels. The ciliary muscles
within the eye and the arrector pili
muscle of the skin are also multiunit. This smooth muscle contains few gap
junctions and the autonomic nervous system innervates each smooth cell and
regulates them like motor units so you can have graded responses. Single unit
smooth muscle lines all the hollow organs and is most common. This type smooth
muscle tends to contract rhythmically, is coupled by numerous gap junctions,
and often exhibits spontaneous action potential. Another nomenclature separates
smooth muscle by contractile pattern. It may contract phasically with rapid contraction and relaxation,
or tonically with slow and sustained contraction.
Smooth muscle in various regions of the vascular tree, the airway and lungs,
kidneys, etc. is different in their expression of ionic channels, hormone
receptors, cell-signaling pathways, and other proteins that determine function.
Smooth muscle-containing tissue often must be stretched, so elasticity is an
important attribute of smooth muscle. Smooth muscle cells may secrete a complex
extracellular matrix containing collagen (predominantly types I and III), elastin, glycoproteins,
and proteoglycans.

These fibers with
their extracellular matrices contribute to the viscoelasticity of these tissues. Smooth muscle also
has specific elastin and collagen receptors to
interact with these proteins.
· Cardiac muscle is also an "involuntary muscle" but is a
specialized kind of muscle found only within the heart.

This is a specialized muscle that, while similar in some
fundamental ways to smooth muscle and skeletal muscle, has a unique structure
and with an ability not possessed by muscle tissue
elsewhere in the body. Cardiac muscle, like other muscles, can contract, but it
can also carry an action potential (i.e. conduct electricity), like the neurons
that constitute nerves. Furthermore, some of the cells
have the ability to generate an action potential, known as cardiac muscle automaticity.
As the muscle
contracts, it propels blood into
the heart and through the blood vessels of the circulatory system. For a human being,
the heart beats about once a second for the entire life of the person, without
any opportunity to rest (Ward 2001). It can adjust quickly to the body's needs,
increasing output from five liters of blood per minute to more than 25 liters per minute
(Ward 2001). The muscles that contract the heart can do so without external
stimulation from hormones or nerves, and it does not fatigue or stop
contracting if supplied with sufficient oxygen and nutrients.

The actions of
cardiac muscle reflect on the remarkable harmony within a body and the
underlying principle that individual entities in nature provide a larger
function. In order for the heart to work properly, and have the necessary waves
of contraction to pump blood, the cardiac cells must fire in intricate
coordination with each other. In doing so, each cell provides a larger function
for the sake of the body, allowing the heart to beat properly, while in turn
being provided essential nutrients by the body. The coordination of the cardiac
cells is essential. Should the cells fire randomly, the heart would not be able
to contract in a synchronized manner and pump blood, and the body (and thus the
cell) would die.
Structure and
functions of myofibril proteins
(myosine, actine,
actomyosine, troponine, tropomyosine).


Actin:
The individual actin protein is called a
"globular" protein
("g-actin") because it is globular, or
ball-like, in appearance. It takes many of these globular proteins
coming together into a long chain to begin to make a microfilament. In
fact, the actin microfilament is two of these chains
of g-actin twisted up together, and the filamentous
form is then called "f-actin." This is
all depicted here in this schematic to the right; the two identical chains of actin are drawn in different colors only so that you can
see how they come together.
You should be able to see how the microfilament can get long, so it should make
sense that it runs along the long axis of the myofilament.
You should also be able to see that the actin
microfilament, although a doublet, is actually rather thin for its length
(which would extend beyond the edges of your monitor)... that's why the actin microfilament has been nicknamed the thin filament. This actin microfilament will also be associated with some other
molecules, called troponin and tropomyosin,
but we won't get to that in detail until next week.
Myosin:
Myosin microfilaments, like actin microfilaments, are
made up of many individual myosin protein molecules. However, the myosin
protein is not globular.
Instead, it has a head and a tail (these regions are indicated in the figure to
the left). And each complete myosin molecule in muscle is actually
composed of two of these head-and-tail molecules twisted around each other.

Note that there
are three different polypeptides that contribute to this overall myosin
protein... that is not important for you to memorize, but you will see it as
you browse through the web, so I have it in this photo; you only need to know
about the head and the tail for this class.
Then, to make the myosin filament, you have to take these doublet myosin
molecules and put them together into large bundles. This big wad of
myosin proteins is then nicknamed the thick
filament. A single thick filament typically has over 200 myosin
molecules in it! So it is really very thick. This is shown in the
figure below (the A with the
circle over it in this figure stands for Angstroms, which are a unit of length
measurement... 1,000,000 Angstroms fit into one micrometer, and 1,000,000
micrometers fit into a meter; you do not have to memorize the dimensions):

Again, the thick
filament runs along the long axis of the myofibril. I found the above
two drawings from educational sites, but that was a couple of years ago and I
have lost the links. I will attempt to find them again!
Arrangement of
thin and thick filaments in a myofibril:
The thin and thick filaments are organized into neat bundles called sarcomeres.

You can read
about sarcomeres in your book. You can also
check out the from
a course at Stanford, and look at the sarcomere link
for "other browsers." I'm just going to give you the basics
here.

Thin filaments
attach at a point called the "Z line" so that they are all lined up with one
another. This is shown in this schematic to the right. The Z line
is the dark line that runs perpendicular to the actin
filaments. Actually, it is simply a lot of sticky proteins that anchor
the actin filaments in place.
The thick filaments run in between the thin filaments. I have put them in
for you to see, but first I had to change the background color of the image so
that both the thick and thin filaments would be readily visible. I also
had to shrink the components down a bit more.

Here is the image
of the thick and thin filaments together:
In order to
fit the thick filaments between the thin, I needed 2 sets of the thin
filaments. Also, it looks like the thick filaments are just floating in
the middle... however, they are anchored by proteins (of the "M line");
I just didn't show that. As you look at this image, the following items
should become easier to understand:
1. A sarcomere
runs from Z-line to Z-line.
2. Sarcomeres
run along the longitudinal axis of the muscle fiber.
3. One sarcomere
connects directly to the next... so I extended the drawing above just a little
bit to help you imagine this:
4. If you look at the sarcomere
from the side , it would look darker in the area where
the myosin runs than in the area where the actin
runs. Because of this, we can talk about light and dark bands. The
dark band, where the myosin runs, is called the A band. The light band,
where the actin runs, is called the I band.
5. How does this relate to the myofibril?
A chain of sarcomeres, like the one I drew
above in point #3, that runs from one end of the muscle fiber to the other end
of the muscle fiber is a myofibril. It is these chains of sarcomeres, or myofibrils, that
are going to allow for contraction, since they are made up of cytoskeletal machinery. Therefore, our muscle fiber
is going to need many of these myofibrils in order to be good at contraction.
Putting the
myofibrils back into the muscle fiber...
First of all, there are hundreds of myofibrils in each muscle fiber.
These are images of cross sections through muscle fibers... you'll see many
dots on the cut edges; each of those dots is a myofibril.
Adjacent myofibrils line up evenly with each other. That means that the
Z-lines of every sarcomere in one myofibril lines up
with the Z-lines of every sarcomere in the adjacent
myofibrils. Because of that, the I bands and the
A bands in all the myofibrils within a muscle fiber are lined up. This is
a difficult point to be able to understand with mental imagery. Take a
look at the image here If you look up again at the drawing
above of the sarcomere, you'll see that the sarcomere runs left and right, but the bands run up and
down. Just like in this photo. This causes the muscle fibers to
look striped, and this appearance is called striated.
We can say that the muscle fibers are striated because they have striations
(stripes).

Take a look at
your textbook Figure 9.5 to see how adjacent myofibrils line up within a muscle
fiber. For the sake of clarity, this figure only shows 9 myofibrils
within the muscle fiber-- but there would be hundreds!
Now if you think back to the last web page, you'll remember that there are cell
membrane invaginations called t-tubules. These t-tubules run into the
muscle fiber at the Z-lines (although your book's Figure 9.5 doesn't really
show it like that).
Mechanism
of muscle’s contraction and relaxation. Role of calcium
and ATP.
When the hypopolarizing stimulus of the spike in the T tubules is
over, calcium ceases to be released by the cisternae
of the sarcoplasmic reticulum and actively pumped
into the longitudinal portion of the reticulum. The Ca pump that pumps Ca from
the cytosol back into the sarcoplasmic
reticulum is an ATPase that is phosporylated
and dephosphorylated during the pumping process. It
pumps two Ca ions for each ATP hydrolyzed. In muscle, the Ca ATPase accounts for nearly 90% of the membrane protein and
therefore is capable of pumping Ca ions rapidly. Typically, the cytosolic Ca concentration is restored to resting levels
within 30 milliseconds. When calcium is removed from the myofibrils, ATP
replaces ADP on the myosin complex and the myosin-actin
bond is broken. Because the muscle is elastic, it will be restored to its
resting length in the absence of a further stimulus to release calcium.
Shortening is an active process; lengthening is a passive process.


A single cycle of
attachment, swivel, and detachment of the myosin head will produce a linear
translation of the myofilaments of about 10 nm. If
all cross-bridges in a myofibril cycle once synchronously, a relative movement
equal to about 1% of the muscle length will occur, but obviously muscles
shorten by more than 1%. The total shortening of a sarcomere
during contraction may exceed 1,000 nm; therefore the relative movement of a
thin and thick filament would be half this amount or 500 nm. To achieve this
magnitude of change in total length when each cross-bridge cycle produces a
10-nm shortening, a minimum of 50 cycles must occur. The flexor muscles of the
human upper arm can contract at the rate of 8 m/sec, during which they can
shorten by as much as 10 cm.
This contraction rate gives a contraction rate for the sarcomere
of 160 nm/msec. If a stroke
of the cross-bridge is taken to be 10 nm, then at this rate there will be a
minimum of 16 strokes/msec.
Thus, the swivel time for the cross-bridge must be of the order of 60 sec.
Calculations for the frog's sartorius muscle, which
can shorten at up to 4 cm/sec, indicate a swivel time of about 1 msec, but this contraction occurs at a lower temperature
than those in mammals. In any case, it is clear that the swiveling of the
cross-bridge must be a fast mechanical process. At the right is an animation
that shows the repeated nature of the process.
The contractile
characteristics and the mechanisms that cause contraction of vascular smooth
muscle (VSM) are very different from cardiac
muscle. VSM undergoes slow, sustained, tonic
contractions, whereas cardiac muscle contractions are rapid and of relatively
short duration (a few hundred milliseconds). While VSM
contains actin and myosin, it does not have
the regulatory protein troponin as is found in the heart.


Furthermore, the
arrangement of actin and myosin in VSM is not organized into distinct bands as it is in
cardiac muscle. This is not to imply that the contractile proteins of VSM are disorganized and not well-developed. They are
actually highly organized and well-suited for their role in maintaining tonic
contractions and reducing lumen diameter.
Contraction in VSM can be initiated by mechanical, electrical, and
chemical stimuli. Passive stretching of VSM can
cause contraction that originates from the smooth muscle itself and is therefore
termed a myogenic response. Electrical depolarization of the VSM cell membrane also elicits contraction, most likely by
opening voltage dependent calcium channels (L-type calcium channels), which
causes an increase in the intracellular concentration of calcium.
Finally, a number of chemical stimuli such as norepinephrine, angiotensin II, vasopressin, endothelin-1, and thromboxane A2 can cause contraction. Each of
these substances bind to specific receptors on the VSM
cell (or to receptors on the endothelium adjacent to the VSM),
which then leads to VSM contraction.


Troponin and tropomyosin are regulatory proteins that allow the muscle
to shorten in the presence of Ca++.
The
mechanism of contraction involves different signal transduction
pathways, all of which converge to increase intracellular calcium.The mechanism by which an increase in intracellular
calcium stimulates VSM contraction is illustrated in
the figure to the right. An increase in free intracellular calcium can
result from either increased flux of calcium into the cell through calcium
channels or by release of calcium from internal stores (e.g., sarcoplasmic reticulum; SR). The free calcium binds
to a special calcium binding protein called calmodulin. Calcium-calmodulin
activates myosin light chain kinase (MLCK), an enzyme that is capable of phosphorylating
myosin light chains (MLC) in the presence of
ATP. Myosin light chains are 20-kD regulatory subunits found on the myosin heads. MLC phosphorylation leads to cross-bridge formation between
the myosin heads and theactin filaments, and hence,
smooth muscle contraction.

Intracellular
calcium concentrations, therefore, are very important in regulating smooth
muscle contraction. The concentration of intracellular calcium depends
upon the balance between the calcium the enters the
cells, the calcium that is released by intracellular storage sites (e.g., SR),
and removal of calcium either back into storage sites or out of the
cell. Calcium is re-sequestered by the SR by a ATP-dependent
calcium pump. Calcium is removed from the cell to the
external environment by either a ATP-dependent
calcium pump or
by the sodium-calcium
exchanger.

Energetic providing of muscle’s work.
ATP is the immediate source of energy for
muscle contraction. Although a muscle fiber contains
only enough ATP to power a few twitches, its ATP "pool" is
replenished as needed. There are three sources of high-energy phosphate to keep
the ATP pool filled.
· creatine
phosphate
· glycogen
· cellular
respiration in the
mitochondria of the fibers.

Creatine phosphate
The phosphate group in creatine
phosphate is attached by a "high-energy" bond like that in ATP.
Creatine phosphate derives its high-energy phosphate
from ATP and can donate it back to ADP to form ATP.
Creatine phosphate + ADP ↔ creatine +
ATP
The
pool of creatine phosphate in the fiber
is about 10 times larger than that of ATP and thus serves as a modest reservoir
of ATP.
Glycogen
Skeletal
muscle fibers contain about 1% glycogen. The muscle fiber can degrade this glycogen by glycogenolysis producing glucose-1-phosphate. This
enters the glycolytic pathway to yield two molecules of ATP
for each pair of lactic acid molecules produced. Not much, but enough to keep
the muscle functioning if it fails to receive sufficient oxygen to meet its ATP
needs by respiration.
However,
this source is limited and eventually the muscle must depend on cellular
respiration.
Cellular
respiration
Cellular
respiration not only is required to meet the ATP needs of a
muscle engaged in prolonged activity (thus causing more rapid and deeper
breathing), but is also required afterwards to enable the body to resynthesize glycogen from the lactic acid produced earlier
(deep breathing continues for a time after exercise is stopped). The body must
repay its oxygen debt.
Most
skeletal muscles contain some mixture of Type I and Type II fibers,
but a single motor unit always contains one type or the other,
never both.
Properties
of White and Red Muscles
The
properties of both red and white muscles are summarized in Table. The
properties of slow muscle fibers make them most suited to extended periods of
contraction where a minimum force is required, e.g., in maintenance of posture.
Fast muscle fibers are better suited to short periods of rapid contraction at
higher forces, e.g., in sprint running. In fact, during exercise training there
may be a differential effect on the two types of muscles. Strength training
leads to hypertrophy of mainly white muscles with conversion of FOG to FG fibers. The number of fibers does not increase, but the
size of fibers and the number of myofibrils do
increase. This increases both the strength and velocity of contraction.
Endurance training apparently affects mainly red muscle fibers, causing an
increase in concentration of the enzymes of oxidative phosphorylation, an
increase in the vascularization of the muscle and
conversion of FG to FOG fibers, but no change in the
ratio of fast to slow fibers and no change in muscle size.
Red
muscles

The
ratio of Type I and Type II fibers can be changed by
endurance training (producing more Type I fibers).
· The action potential that triggers the
heartbeat is generated within the heart itself. Motor nerves (of the autonomic nervous
system) do run
to the heart, but their effect is simply to modulate — increase or decrease —
the intrinsic rate and the strength of the heartbeat. Even if the nerves are
destroyed (as they are in a transplanted heart), the heart continues to beat.
· The action potential that drives
contraction of the heart passes from fiber to fiber through gap junctions.
· Significance: All the fibers contract in a synchronous wave that sweeps from the
atria down through the ventricles and pumps blood out of the heart. Anything
that interferes with this synchronous wave (such as damage to part of the heart
muscle from a heart attack) may cause the fibers of
the heart to beat at random — called fibrillation.
Fibrillation is the ultimate cause of most deaths and its reversal is the
function of defibrillators that are part of the equipment in ambulances,
hospital emergency rooms, and — recently — even on U.S. air lines.
· The refractory period in heart muscle is longer than the period it takes for the
muscle to contract (systole) and relax (diastole).
· Cardiac muscle has a much richer
supply of mitochondria than skeletal muscle. This reflects its greater
dependence on cellular respiration for ATP.
· Cardiac muscle has little glycogen and
gets little benefit from glycolysis when the supply
of oxygen is limited.
· Thus anything
that interrupts the flow of oxygenated blood to the heart leads quickly to
damage — even death — of the affected part. This is what happens in heart attacks.
Below:
the human heart, with a schematic view of the pathway of blood through the
lungs and internal organs. Oxygenated blood is shown in red; deoxygenated blood
in blue. Note that the blood draining the stomach, spleen, and intestines
passes through the liver before it is returned to the heart. Here surplus or
harmful materials picked up from those organs can be removed before the blood
returns to the general circulation.
Peculiarities of metabolism in cardiac muscle.
Cardiac muscle
Structurally,
cardiac muscle is similar to skeletal muscle in that it is striated, having
both thick and thin filaments. It has a well-developed T tubule system,
although the sarcoplasmic reticulum is not as large
or as extensive as in skeletal muscle. Unlike those in skeletal muscle, the
triads of cardiac muscle of humans are located at the Z line, giving only one
per sarcomere. The mechanism of
excitation-contraction coupling is the same as for skeletal muscle: The
membrane action potential leads to an increase in Ca++ around the myofilaments
that activates myosin-ATPase and leads to sliding of
the thin and thick filaments. The source of the calcium is different in cardiac
muscle. Because the sarcoplasmic reticulum is poorly
developed, it cannot sequester the large amount of calcium that skeletal muscle
can. Therefore, much of the calcium for contraction must come from
extracellular sources; it comes in during the action potential.
There
are a large number of different kinds of cells in cardiac muscle. These include
cells of the sinoatrial node, the atrioventricular
node, the atrium, the bundle of His, and the ventricle, each with a differently
shaped action potential. The details of these differences are beyond the scope
of this treatment. For our purposes, it is convenient to distinguish two kinds
of cardiac muscle cells: pacemaker cells, like the Purkinje fibers, and
contractile cells. Examples of a Purkinje fiber action potential (A) and a
contractile cell action potential (B) Both action potentials are much longer in
duration than spikes in nerve cells and skeletal muscle cells, 0.5 sec compared
to 0.5 to 5.0 msec. The hypopolarizing phase of the
Purkinje fiber's action potential is not different from that in skeletal
muscle, and it appears to have the same ionic mechanism, i.e., a dramatic
increase in sodium conductance. The
contractile cell's action potential has two rising phases, a rapidly rising
phase, like that in the Purkinje fiber, and a more slowly rising phase. The
fast phase has the same mechanism as the rising phase of Purkinje fiber action
potentials, but the slower phase is the result of a slow inward current,
carried mostly by Ca++. Calcium current activation occurs at a more hypopolarized level of the membrane potential than does sodium activation, and the inactivation of the calcium
current is less rapid by about two orders of magnitude.
The
long plateau of the action potential in cardiac muscle serves two functions: It
provides a more prolonged contraction without resorting to tetanus, and it
provides a longer refractory period to prevent the heart from contracting prematurely.
This plateau is produced by a number of factors, the most important of which is
a decrease in potassium conductance with hypopolarization, followed by a slowly developing increase
that brings the potassium conductance to a final value just slightly greater
than resting levels in Purkinje fibers and to resting levels in contractile
cells in about 300 msec. A change in membrane conductance with changes in
membrane potential is called rectification by biophysicists. This change in
potassium conductance is called anomalous rectification.
Cardiac
muscle behaves much like skeletal muscle, but it exerts a passive tension when
stretched at much shorter lengths. In fact, when the muscle is stretched from a
length even shorter than resting length, there is a resistance to the stretch.
In other words, cardiac muscle experiences elastic tension even at resting
length (skeletal muscle does not). In addition, the maximum developed tension
in cardiac muscle occurs, not at the resting length, but when it is stretched
beyond resting length. The result is that when more blood returns to the heart
from the veins, the muscle fibers of the heart will be stretched more, and the
blood will automatically be pumped out more forcefully than when the heart is
just normally full. This is the basis of the Frank-Starling mechanism in the
heart.
Diagnostic
significance of determination of creatin, creatinin and creatin phosphokinase’s activity in biological fluids
Creatinine
It
is used to find out whether your kidneys are working normally. A combination of
blood and urine creatinine levels may be used to
calculate a "creatinine clearance". This
measures how effectively your kidneys are filtering small molecules like creatinine out of your blood.
Urine creatinine may
also be used with a variety of other urine tests as a correction factor. Since
it is produced and removed at a relatively constant rate, the amount of creatinine in urine can be compared to the amount of
another substance being measured. Examples of this are when creatinine
is measured with protein to calculate a urine protein/creatinine
ratio (UP/CR) and when it is measured with microalbumin
to calculate microalbumin/creatinine ratio (also
known as albumin/creatinine ratio, ACR). These tests are used to evaluate kidney function as
well as to detect other urinary tract disorders.
Serum creatinine
measurements along with age, weight, and gender are used to calculate the
estimated glomerular filtration rate (eGFR), which is used as a screening test to look for
evidence of kidney damage.
Creatinine may be part of a routine blood test, widely used
when someone has non-specific health complaints, or when your doctor suspects
your kidneys are not working properly.
Some signs and symptoms of kidney dysfunction
include:
Fatigue,
lack of concentration, poor appetite or trouble sleeping
Swelling
or puffiness, particularly around the eyes or in the face, wrists, abdomen,
thighs or ankles
Urine
that is foamy, bloody, or coffee-coloured
A
decrease in the amount of urine
Problems
urinating, such as a burning feeling or abnormal discharge during urination, or
a change in the frequency of urination, especially at night
Mid-back
pain (flank), below the ribs, near where the kidneys are located
High
blood pressure
The
test is also used to monitor treatment of kidney disease or to monitor kidney
function while you are on certain drugs.
What
does the test result mean?
Increased
creatinine levels in the blood suggest diseases that
affect kidney function. These can include:
damage to or swelling of blood vessels in the kidneys (glomerulonephritis) caused by, for example, infection or
autoimmune diseases bacterial infection of the kidneys (pyelonephritis)
death of cells in the kidneys’ small tubes (acute tubular
necrosis) caused, for example, by drugs or toxins
prostate disease, kidney stone, or other causes of urinary
tract obstruction; or
reduced blood flow to the kidney due to shock, dehydration,
congestive heart failure, atherosclerosis, or complications of diabetes
Creatinine blood
levels can also increase temporarily as a result of muscle injury and are
generally slightly lower during pregnancy.
Low levels of creatinine
are not common and are not usually a cause for concern. As creatinine
levels are related to the amount of muscle the person has, low levels may be a
consequence of decreased muscle mass (such as in the elderly), but may also be
occasionally found in advanced liver disease.
Random urine creatinine
levels have no standard reference ranges. They are usually used with other
tests to reference levels of other substances measured in the urine. Some
examples include the microalbuminuria test and urine
protein test.
Since
creatinine levels are in proportion to muscle mass,
women tend to have lower levels than men.
In
general, creatinine levels will stay the same if you
eat a normal diet. However, eating large amounts of meat may cause short-lived
increases in blood creatinine levels. Taking creatine supplements may also increase creatinine.
There
are a few drugs that interfere with the creatinine
test, although there are some drugs that can cause some impairment in kidney
function. Your creatinine levels may be monitored if
you are taking one of these drugs.
Diagnostic
significance of determination of creatin phosphokinase’s activity(CK)
CK
is often determined routinely in a medical laboratory. It is also determined
specifically in patients with chest pain or if acute renal failure is
suspected. Normal values are usually between 60 and 400 IU/L,
where one unit is enzyme activity, more specifically the amount of enzyme that
will catalyze 1 μmol of substrate per minute
under specified conditions (temperature, pH, substrate concentrations and
activators. This test is not specific for the type of CK that is elevated.
Elevation
of CK is an indication of damage to muscle. It is therefore indicative of
injury, rhabdomyolysis, myocardial infarction, myositis and myocarditis. The use
of statin medications, which are commonly used to
decrease serum cholesterol levels, may be associated with elevation of the CPK level in about 1% of the patients taking these
medications, and with actual muscle damage in a much smaller proportion.
There
is an inverse relationship in the serum levels of T3 and CK in thyroid disease.
In hypothyroid patients, with decrease in serum T3 there is a significant
increase in CK. Therefore, the estimation of serum CK is considered valuable in
screening for hypothyroid patients.
Lowered
CK can be an indication of alcoholic liver disease and rheumatoid arthritis.
Isoenzyme determination has been used extensively as an
indication for myocardial damage in heart attacks. Troponin
measurement has largely replaced this in many hospitals, although some centers
still rely on CK-MB.
Biochemistry of connective tissue.
Connective tissues are responsible for the form and shape of the animal
body and, in addition, provide protection for vital organs and facilitate
locomotion. The term connective tissue is also applied in a more restricted
sense to structures such as dermis, tendons, fascia, bone, cartilage, and the
capsules of the joint. All cells, however, make contacts with surrounding
structures that involve connective tissues as components of the extracellular
matrix. The matrix possesses chemical, physical, and mechanical properties
uniquely suited to the function of tissues and organs of which the cells are a
part. The extracellular matrix may be rigid (e.g., bone), elastic (e.g., blood
vessel walls), compressible (e.g., cartilage), or liquid (e.g., synovial
fluid). Most connective tissue matrices derive these properties by virtue of
the content of fibrillar proteins, nonfibrillar macromolecules, and low molecular weight
proteins and electrolytes.

The
properties of the matrix are therefore determined predominantly by the function
of cells, specific for each tissue, which are responsible for synthesis of the
matrix components. Many of the functions of the component cells, in turn, are
influenced by the character of the extracellular matrix. The properties of
connective tissues are also influenced by their relationships to the vascular
system from which critical components are derived, such as water, electrolytes,
and proteins. Indeed, the walls of blood vessels may themselves be considered
as connective tissues. However, although some connective tissues are highly
vascular (e.g., bone), others are essentially avascular
(e.g., cartilage).
You
will be examining several types of connective tissues. General
characteristics of connective tissue include; vascularization, lots of intercellular matrix
(space between cells), and fibers.
There
are three types of fibers found commonly in connective tissue; collagenous,
elastic and reticular.
The
intercellular matrix can vary in consistancy, from a
solid to a fluid. The number of cells in connective tissue when compared
to the number of cells in epithelial tissue is considerably less.
The
major functions of connective tissue:
is to protect, to support, to transport, to store, and
the obvious one "to connect" one tissue type to another.
The
most common cell types are fibroblasts

which produce fibres and other intercellular materials.

Classification
of Connective Tissue
I. Connective Tissue Proper - encompasses all organs and body cavities connecting
one part with another and, equally important, separating one group of cells
from another. This is a very large and diverse group of tissues and
includes adipose tissue (fat), areolar (loose)
tissue, and dense regular tissue, among others.

adipose tissue

II. Specialized Connective Tissues -- this group includes cartilage, bone, and
blood. Cartilage and bone form the skeletal framework of the body while
blood is the vascular (transport) tissue of animals.



cartilage blood

Cartilage
is a somewhat elastic, pliable, compact type of connective tissue. It is
characterized by three traits: lacunae,
chondrocytes, and
a rigid matrix. The matrix is a firm gel material that contains
fibres and other substances. There are three basic types of cartilage in the
human body: hyaline cartilage, elastic cartilage and fibrocartilage.
In this laboratory, you will examine the most common type of cartilage, the hyaline cartilage. Most of the
skeleton of the mammalian fetus is composed of
hyaline cartilage.

As
the fetus ages, the cartilage is gradually replaced
by more supportive bone. In the mammalian adult, hyaline cartilage is
mainly restricted to the nose, trachea, bronchi, ends of the ribs, and the
articulating surfaces of most joints. The function of the hyaline cartilage is
to provide slightly flexible support and reduce friction within joints. It also
provides structural reinforcement.
The matrix appears as a smooth, solid, blue or
pink-coloured substance. Fine protein fibres,
are embedded in the matrix, but they are not visible with the light microscope
since they do not stain well. Locate the large cartilage cells called chondrocytes,
which are trapped within the matrix in spaces called lacunae (singular, lacuna).
Cartilage
is a non-vascular tissue. As such, the chondrocytes
rely on blood vessels in the tissue surrounding the cartilage for nutrient
supply and waste removal. Considering this structural feature, can you
make a general comment as to the potential "thickness" of cartilage?

Schematic representation of hyaline cartilage.

Microscopic view of hyaline cartilage.

I. Connective tissue
proper
a) Areolar (Loose) Connective Tissue

Areolar connective tissue is the most widespread connective
tissue of the body. It is used to attach the skin to the underlying
tissue. It also fills the spaces between various organs and thus holds them in
place as well as cushions and protects them. It also surrounds and supports the
blood vessels.

areolar (loose) tissue
The fibres of areolar connective tissue are arranged
in no particular pattern but run in all directions and form a loose network in
the intercellular material. Collagen(collagenous) fibres are
predominant. They usually appear as broad pink bands. Some elastic fibres, which appear as thin, dark
fibres are also present.
The cellular elements, such as fibroblasts,
are difficult to distinguish in the areolar
connective tissue. But, one type of cells - the mast cells are usually visible. They have course, dark-staining granules in their
cytoplasm. Since the cell membrane is very delicate it frequently
ruptures in slide preparation, resulting in a number of granules free in the
tissue surrounding the mast cells. The nucleus in these cells is small,
oval and light-staining, and may be obscured by the dark granules.
Schematic representation of the areolar
connective tissue.

Microscopic view of areolar connective
tissue.
Loose or areolar connective tissue.
Thick pink bands are the protein collogen, while the
thin dark threads are the protein elastin.
Adipose
Connective Tissue

The cells of adipose (fat) tissue are characterized by a large internal fat
droplet, which distends the cell so that the cytoplasm is reduced to a thin
layer and the nucleus is displaced to the edge of the cell. These cells
may appear singly but are more often present in groups. When they
accumulate in large numbers, they become the predominant cell type and form
adipose (fat) tissue.
Adipose
tissue, in addition to serving as a storage site for fats (lipids), also pads
and protects certain organs and
regions of the body. As well, it forms an insulating layer under the skin
which helps regulate body temperature.
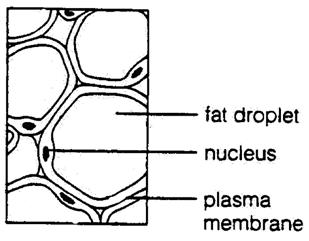
Schematic representation of the adipose connective tissue.
Dense connective tissue is characterized by an abundance of fibres with fewer
cells, as compared to the
loose connective tissue. It is also called fibrous or collagenous
connective tissue because of the abundance of collagen
(collagenous) fibres. Little intercellular substance
is present. Furthermore, in this tissue type, the fibres are organized in a
regular, parallel pattern. Hence, the name – dense regular
(fibrous or collagenous) connective tissue.
In addition to the tendons, this type
of tissue is also found in ligaments. Hence, the function of this tissue is to
anchor skeletal muscle to bone, to attach bone to bone as well as to stabilize
the bones within a joint. On your slide, note that the collagen fibres are
parallel to one another. Fibroblasts are the only cells visible, and are
arranged in rows between the fibres. These fibroblasts function to lay down or create the fibres of the tissue.

Schematic representation of dense regular connective tissue.
Structure and functions of collagen.
Collagen
is a fibrous protein that consists of three α-chains (which are not α-helices, 'helpfully' enough), which form a rope-like triple
helix, providing tensile strength to the ECM.


Collagen.
α chains contain GXY
repeats: glycine (G) is small, and is the only amino
acid that fits in the crowded interior of the triple helix. X is usually proline, which destabilises the
formation of a simple α-helix and Y is usually hydroxyproline; the hydrogen-bonding between the OH groups
on this hydroxylated form of proline
stabilises the triple helix. Hydroxyproline
is formed post-translationally by the action of proline hydroxylase. This enzymes has a vitamin-C cofactor, which explains the
symptoms of scurvy: tissues containing collagen (gums, skins, capillaries) are
weakened, because the unhydroxylated collagen is
destroyed without being secreted.
The
synthesis of collagen molecules begins on the RER as
with all secreted proteins. The pro-α-chains are made on the RER, and are
hydroxylated and glycosylated
(on hydroxylysines) in the Golgi. Procollagen
forms from three α-chains, and possesses terminal 'propeptides'. This procollagen is
then secreted from vesicles, and undergoes proteolysis at its ends in the
extracellular space, to form mature 100 nm long collagen molecules.

Collagen
molecules are then crosslinked into fibrils:
oxidative deamination of hydroxylysine
and lysine forms reactive aldehyde groups, which link
molecules together (and also linkα-chains
together too).

Collagen
fibrils then self-assemble into fibres, which form
characteristically straited 'ropes' under EM. Collagen fibrils have a service life of 10 years or so:
most enzymes turn-over in about an hour.

Collagen
comes in many different types. Type I collagen is the most common fibrillar collagen (90%), and is found in skin, bone,
tendons, etc. Type II
collagen provides similar tensile strength to cartilage.
Other
sorts of collagen do not form fibres: type IX collagen (and type XII) are fibril-associated collagens,
which link type I (or type II) collagen fibrils together. They are more
flexible than fibrillar collagens because the GXY-repeats of their α-chains are interrupted more
frequently by other amino acids. Type IV and VII collagens are network-forming
collagens; they form a meshwork, particularly in basal lamina.

Collagen
provides tensile strength to the ECM, but other
proteins provide other properties. Elastin forms
elastic fibres, which give the ECM
its elasticity (surprise!). Elastin is secreted as
the tropoelastin precursor. It is then cross-linked
in similar way to collagen to form a stretchy net of elastin.
Elastic fibres are coated with fibrillin
microfibrils. Defects in the
fibrillin-1 gene cause Marfan syndrome, which is characterised by weak elastic tissue, causing long fingers,
pigeon chest, and the aorta to be very weak. Abraham Lincoln is thought to
have had Marfan syndrome.

Biosynthesis of collagen.
Amino
acids
Collagen
has an unusual amino acid composition and sequence:
·
Glycine (Gly) is
found at almost every third residue
·
Proline (Pro) makes up about 9% of collagen
·
Collagen
contains two uncommon derivative amino acids not directly inserted during translation.
These amino acids are found at specific locations relative to glycine and are modified post-translationally
by different enzymes, both of which require vitamin
C as a cofactor.
o
Hydroxyproline (Hyp),
derived from proline.
o
Hydroxylysine,
derived from lysine.
Depending on the type of collagen, varying numbers of hydroxylysines
have disaccharides attached to them.
Collagen
I formation

Most
collagen forms in a similar manner, but the following process is typical for
type I:
1. Inside the
cell
1. Three peptide chains are formed (2 alpha-1 and 1
alpha-2 chain) in ribosomes along the Rough Endoplasmic Reticulum (RER). These
peptide chains (known aspreprocollagen) have registration peptides on each end; and a signal peptide is also attached to each
2. Peptide chains are sent into the lumen
of the RER
3. Signal Peptides are cleaved inside the
RER and the chains are now known as procollagen
4. Hydroxylation of lysine and proline amino acids occurs inside the lumen.
This process is dependent on Ascorbic Acid (Vitamin C) as a cofactor
5. Glycosylation of specific hydroxylated amino acid occurs
6. Triple helical structure is formed
inside the RER
7. Procollagen is shipped to
the golgi apparatus, where it is packaged
and secreted by exocytosis
2. Outside the
cell
1. Registration peptides are cleaved and tropocollagen is formed by procollagen peptidase.
2. Multiple tropocollagen
molecules form collagen fibrils, and multiple collagen
fibrils form into collagen fibers
3. Collagen is attached to cell membranes
via several types of protein, including fibronectin and integrin.
Synthetic
pathogenesis
Vitamin
C deficiency causes scurvy, a serious and painful disease in which defective collagen prevents
the formation of strong connective tissue.

Gums deteriorate and bleed, with loss of teeth; skin discolors,
and wounds do
not heal. Prior to the eighteenth century, this condition was notorious among
long duration military, particularly naval, expeditions during which
participants were deprived of foods containing Vitamin C. In the human body, a
malfunction of the immune system, called anautoimmune disease, results in an immune
response in which healthy collagen fibers are systematically destroyed with
inflammation of surrounding tissues. The resulting disease processes are called Lupus erythematosus, and rheumatoid arthritis, or collagen tissue
disorders.
Many
bacteria and viruses have virulence factors which destroy collagen or interfere
with its production.
Elastin – main
protein of elastic fibrils, structure and biological role.
The
two most common types of fibres are: collagen
(collagenous) and elastic. Collagen fibres are for
strength while the elastic ones are for elasticity of the tissue. Both the
cells and the fibres are embedded in the intercellular
substance. The consistency of this substance is highly variable from gelatin-like to a much more rigid material.

Elastic
Fibers

The proportions of the cells, fibres, and intercellular substance vary, depending on a
particular nature and function of the connective tissue. For example, a strong
connective tissue needs a greater proportion of the collagen fibres and fewer
cells. An example would be a dense regular connective tissue, which is found in
tendons and ligaments. On the other hand, a connective tissue composed of
mostly cells would not be very strong. An example would be an adipose (fat)
connective tissue.



Elastic fibers are made of elastin
and are "stretchable."

Reticular
Fibers
Reticular fibers join connective tissues to other
tissues.
Structure and functions of proteoglycans.
Metabolism of proteoglycans.
.
The extracellular matrix occupies the space between cells.
Many of the components of the extracellular matrix are connected to proteins of
the cytoskeleton by transmembrane proteins.
The
matrix is a complex network of different combinations of collagens, proteoglycans (PG), hyaluronic
acid, laminin, fibronectin,
and many other glycoproteins including proteolytic enzymes involved in degradation and remodeling
of the extracellular matrix.
The
matrix plays an important structural and functional role in multicellular
organisms. Fibres within the extracellular matrix are
collagen fibres, reticular fibres,
and elastic fibres. In the dermis of the skin, bone,
tendon, organ capsules and many other areas collagen fibres
mainly consist of collagen type 1, which constitutes approximately 90 % of the
body collagen. Collagen fibers in cartilage consist of collagen type 2. The
basement membrane of epithelila consists of collagen type 4.
Reticular fibres are made mainly of collagen type 3
and form a mesh-like structure. In most tissues these fibers are produced by fibroblasts.
The reticular fibers in hematopoietic and lymphatic tissue are made by reticular cells.
Reticular fibers around peripheral nerves are produced by Schwann cells. Smooth muscle cells also produce
reticular fibers. Elastic fibres form a
three-dimensional network interwoven with collagen fibres.
They consist of elastin and microfibrils
and are produced by fibroblasts in most cases. Smooth muscle cells produce these
fibers in elastic arteries.
The
extracellular matrix is more than a scaffold that fills extracellular spaces.
Many of its components are engaged in processes mediating cell-to-cell interactions . In many instances the capacity of a cell to
proliferate, differentiate, and to express specialized functions intimately
depends on the presence and maintenance of an intact extracellular matrix (see,
for example: hematopoiesis).
Components of the extracellular matrix are involved also in the process of anoikis,
a special form of programmed cell
death.
Some
of the matrix components, in particular the proteoglycans,
function as modulators of the biological activities of growth factors.
The organization composition, and physical properties the extracellular
environment are also essential for the modulation of functions of endothelial cells during angiogenesis.
Proteoglycans are proteins modified by glycosaminoglycans (abbr. GAG, called also mucopolysaccharides). Glycosaminoglycans are long-chain compounds made up of
hundreds or less repeating disaccharide units. One of the sugars in each
disaccharide unit is a hexosamine (glycosamine). The four main types consist mainly of
sulfated heparan sulfate/heparin, chondroitin sulfate/dermatan,
keratan sulfate, and the non-sulfated glycosaminoglycan hyaluronic acid. Hyaluronic
acid is an extremely long and rigidglycosaminoglycan containing several thousand sugars
but no protein core. Linker molecules join proteoglycans to hyaluronic
acid. Many proteoglycans contain a core protein which links
them to the cellular membrane.
The combination of a core protein and a specific glycosaminoglycan generates a unique proteoglycan
with a precise developmental pattern. In addition, Glycosaminoglycans are negatively charged compounds and
therefore bind unspecifically to many other
substances, including growth factors.
Some proteins are known to interact specifically withglycosaminoglycans.
The interactions between glycosaminoglycans and cytokines are one of the important mechanisms
underlying communication processes between cells that are mediated by secreted and
locally acting factors.
Mucopolysaccharidoses and collagenoses,
their biochemical diagnostics
Mucopolysaccharidoses are a group of metabolic
disorders caused by the absence or malfunctioning of lysosomal enzymes
needed to break down molecules called glycosaminoglycans - long chains of sugar
carbohydrates in each of our cells
that help build bone,
cartilage,
tendons,
corneas,
skin
and connective
tissue. Glycosaminoglycans (formerly called mucopolysaccharides) are also found in the fluid that
lubricates our joints.
People with a
mucopolysaccharidosis disease either do not produce
enough of one of the 11 enzymes required to break down these sugar chains into
simpler molecules, or they produce enzymes that do not work properly. Over
time, these glycosaminoglycans collect in the cells,
blood and connective tissues. The result is permanent, progressive cellular
damage which affects appearance, physical abilities, organ and system
functioning, and, in most cases, mental development.
The mucopolysaccharidoses are part of the lysosomal storage disease family, a group of more
than 40 genetic disorders that result when a specific organelle in our body's
cells – the lysosome – malfunctions. The lysosome is commonly referred to as the cell’s recycling
center because it processes unwanted material into substances that the cell can
utilize. Lysosomes break down this unwanted matter
via enzymes, highly specialized proteins essential for survival. Lysosomal disorders like mucopolysaccharidosis
are triggered when a particular enzyme exists in too small an amount or is
missing altogether.
The mucopolysaccharidoses
share many clinical features but have varying degrees of severity. These
features may not be apparent at birth but progress as storage of glycosaminoglycans affects bone, skeletal structure,
connective tissues, and organs. Neurological complications may include damage
to neurons
(which send and receive signals throughout the body) as well as pain
and impaired motor function. This results from compression of nerves
or nerve roots in the spinal
cord or in the peripheral
nervous system, the part of the nervous
system that connects the brain
and spinal
cord to sensory organs such as the eyes and to other organs, muscles,
and tissues throughout the body.
Depending on the mucopolysaccharidosis
subtype, affected individuals may have normal intellect or have cognitive
impairments, may experience developmental delay, or may have severe behavioral
problems. Many individuals have hearing loss, either conductive (in which
pressure behind the ear drum causes fluid from the lining of the middle ear to
build up and eventually congeal), neurosensitive (in
which tiny hair cells in the inner ear are damaged), or both. Communicating
hydrocephalus — in which the normal reabsorption of
cerebrospinal fluid is blocked and causes increased pressure inside the head —
is common in some of the mucopolysaccharidoses.
Surgically inserting a shunt
into the brain can drain fluid. The eye's cornea
often becomes cloudy from intracellular storage, and glaucoma
and degeneration of the retina also may affect the patient's
vision.
Physical symptoms generally include coarse or rough facial features
(including a flat nasal bridge, thick lips, and enlarged mouth and tongue),
short stature with disproportionately short trunk (dwarfism),
dysplasia
(abnormal bone size and/or shape) and other skeletal irregularities, thickened
skin, enlarged organs such as liver (hepatomegaly) or spleen (splenomegaly), hernias,
and excessive body hair growth. Short and often claw-like hands, progressive
joint stiffness, and carpal
tunnel syndrome can restrict hand mobility and function. Recurring
respiratory infections are common, as are obstructive airway disease and
obstructive sleep
apnea. Many affected individuals also have heart disease, often
involving enlarged or diseased heart valves.
Another lysosomal storage
disease often confused with the mucopolysaccharidoses
is mucolipidosis. In this disorder, excessive amounts
of fatty materials known as lipids
(another principal component of living cells) are stored, in addition to
sugars. Persons with mucolipidosis may share some of
the clinical features associated with the mucopolysaccharidoses
(certain facial features, bony structure abnormalities, and damage to the
brain), and increased amounts of the enzymes needed to break down the lipids
are found in the blood.
Seven distinct clinical types and numerous subtypes of the mucopolysaccharidoses have been identified. Although each mucopolysaccharidosis (MPS) differs clinically, most
patients generally experience a period of normal development followed by a
decline in physical and/or mental function.
Diagnosis often can be
made through clinical examination and urine tests (excess mucopolysaccharides
are excreted in the urine). Enzyme assays (testing a variety of cells or body
fluids in culture for enzyme deficiency) are also used to provide definitive
diagnosis of one of the mucopolysaccharidoses.
Prenatal diagnosis using amniocentesis
and chorionic
villus sampling can verify if a fetus either
carries a copy of the defective gene or is affected with the disorder. Genetic
counseling can help parents who have a family history of the mucopolysaccharidoses determine if they are carrying the
mutated gene that causes the disorders.
connective tissue diseases
- any of various diseases or abnormal
states (as rheumatoid arthritis, systemic lupus erythematosus, polyarteritis nodosa, rheumatic fever, and dermatomyositis)
characterized by inflammatory or degenerative changes in connective tissue—called also collagen disease, collagenolysis,
collagen vascular disease
Structure and functions of sarcoplasma
proteins (Myogene, Myoglobine,
Myoalbumine)
The sarcoplasm of a muscle
fiber is comparable to the cytoplasm of other cells, but it houses unusually
large amounts of glycosomes (granules of stored
glycogen) and significant amounts of myoglobin, an
oxygen binding protein. The calcium concentration in sarcoplasma
is also a special element of the muscular fiber by means of which the
contractions takes place and regulates.
It contains mostly myofibrils (which are composed of sarcomeres), but its contents are otherwise comparable to
those of the cytoplasm of other cells. It has a Golgi apparatus, near the
nucleus, mitochondria just on the inside of the cytoplasmic
membrane or sarcolemma, as well as a smooth
endoplasmic reticulum organized in an extensive network.
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of
vertebrates in general and in almost all mammals. It is related to hemoglobin,
which is the iron- and oxygen-binding protein in blood, specifically in the red
blood cells. The only time myoglobin is found in the
bloodstream is when it is released following muscle injury. It is an abnormal
finding, and can be diagnostically relevant when found in blood.
Myoglobin (abbreviated
Mb) is a single-chain globular protein of 153 or 154 amino acids, containing a heme (iron-containing porphyrin)
prosthetic group in the center around which the remaining apoprotein
folds. It has eight alpha helices and a hydrophobic core. It has a molecular
weight of 17,699 daltons (with heme),
and is the primary oxygen-carrying pigment of muscle tissues. Unlike the
blood-borne hemoglobin, to which it is structurally related, this protein does
not exhibit cooperative binding of oxygen, since positive cooperativity
is a property of multimeric/oligomeric proteins only.
High concentrations of myoglobin in muscle cells
allow organisms to hold their breaths longer. Diving mammals such as whales and
seals have muscles with particularly high myoglobin
abundance.
Myoglobin was the
first protein to have its three-dimensional structure revealed. In 1958, John
Kendrew and associates successfully determined the structure of myoglobin by high-resolution X-ray crystallography. For
this discovery, John Kendrew shared the 1962 Nobel Prize in chemistry with Max
Perutz. Despite being one of the most studied proteins in biology, its true
physiological function is not yet conclusively established: mice genetically
engineered to lack myoglobin are viable, but showed a
30% reduction in cardiac systolic output. They adapted to this deficiency
through hypoxic genetic mechanisms and increased vasodilation.
In humans myoglobin is encoded by the MB gene.
Meat color
Myoglobin forms
pigments responsible for making meat red. The color that meat takes is partly
determined by the oxidation states of the iron atom in myoglobin
and the oxygen species attached to it. When meat is in its raw state, the iron
atom is in the +2 oxidation state, and is bound to a dioxygen
molecule (O2). Meat cooked well done is brown because the iron atom is now in
the +3 oxidation state, having lost an electron, and is now coordinated by a
water molecule. Under some conditions, meat can also remain pink all through
cooking, despite being heated to high temperatures. If meat has been exposed to
nitrites, it will remain pink because the iron atom is bound to NO, nitric
oxide (true of, e.g., corned beef or cured hams). Grilled meats can also take
on a pink "smoke ring" that comes from the iron binding to a molecule
of carbon monoxide. Raw meat packed in a carbon monoxide atmosphere also shows
this same pink "smoke ring" due to the same coordination chemistry.
Notably, the surface of this raw meat also displays the pink color, which is
usually associated in consumers' minds with fresh meat. This artificially
induced pink color can persist in the meat for a very long time, reportedly up
to one year. Hormel and Cargill are both reported to use this meat-packing
process, and meat treated this way has been in the consumer market since 2003. Myoglobin is found in Type I muscle, Type II A and Type II
B, but most texts consider myoglobin not to be found
in smooth muscle.
Role in disease
Myoglobin is released
from damaged muscle tissue (rhabdomyolysis), which
has very high concentrations of myoglobin. The
released myoglobin is filtered by the kidneys but is
toxic to the renal tubular epithelium and so may cause acute renal failure. It
is not the myoglobin itself that is toxic (it is a protoxin) but the ferrihemate
portion that is dissociated from myoglobin in acidic
environments (e.g., acidic urine, lysosomes).
Myoglobin is a
sensitive marker for muscle injury, making it a potential marker for heart
attack in patients with chest pain. However, elevated myoglobin
has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cTnT, ECG, and clinical signs
should be taken into account to make the diagnosis.
Structure and bonding
Myoglobin contains a porphyrin ring with an iron center. There is a proximal histidine group attached directly to the iron center, and a
distal histidine group on the opposite face, not bonded
to the iron.
Many functional models of myoglobin
have been studied. One of the most important is that of picket fence porphyrin by James P. Collman.
This model was used to show the importance of the distal prosthetic group. It
serves three functions:
To form hydrogen bonds with the dioxygen
moiety, increasing the O2 binding constant
To prevent the binding of carbon
monoxide, whether from within or without the body. Carbon
monoxide binds to iron in an end-on fashion, and is hindered by the presence of
the distal histidine, which forces it into a bent
conformation. CO binds to heme 23,000 times better
than O2, but only 200 times better in hemoglobin and myoglobin.
Oxygen binds in a bent fashion, which can fit with the distal histidine.
To prevent irreversible dimerization
of the oxymyoglobin with another deoxymyoglobin
species
myogen - proteins extracted from skeletal muscle with cold water, largely the
enzymes promoting glycolysis; from the residue,
alkaline 0.6 mol L-1 KCl extracts actin
and myosin as actomyosin, with myosin further
separable into two meromyosins by proteinase
treatment.
Synonym(s): myosinogen
Proteins of the Myofilaments
The biochemical basis of muscle
activity is related to the
enzymatic and physical properties of actin, myosin,
and the accessory
proteins that constitute the thin and thick
filaments. The following discussion summarizes the key protein components
of the myofilaments
and their ATP-dependent interactions, which produce contractile
activity.
The proteins of the
thin and thick filaments can be separated
into actin, myosin, and 6 accessory
proteins. The accessory proteins are α-actinin, β-actinin, tropomyosin,
troponin, C protein, and M line protein.
Solubilized myosin molecules are long
thin (fibrous) proteins with a molecular weight of about 500,000 daltons.
Each molecule is made
up of 6 subunits,
2 very large, heavy chains (HC),
and 4 smaller, light chains (LC).
In a given muscle fiber the
2 large subunits are identical, although there are different HC
isoforms in different types of muscle fibers.
Heavy chains contain a long linear C-terminal α-helical domain (1,300 amino acids) and a prominent
globular N-terminal domain of about
800 amino acids. The two HC,
α-helical domains are helically interwound, giving the molecules
a long, rigid superhelical structure with 2 globular headpieces. A complete myosin molecule also contains 4 relatively small proteins which are associated with the globular
headpieces. These small proteins, of molecular weight
16,000–24,000 daltons, are known as alkali
light chains (LC1 or LC3) and DTNB
light chains (LC2). Each myosin molecule
contains 2 subunits of LC2, 1 associated with each HC
globular domain. Each of the
globular domains also contains a subunit of either
LC1 or LC3, with the proportions of LC1 and LC3 in the myosin
molecules varying in myosins from
cardiac, skeletal, embryonic, and smooth muscle. All light chains
bind Ca2+ with high affinity, are phosphorylated by myosin light
chain kinase (MLCK), and generally
serve in the regulation of myosin's ATPase
activity and its assembly into
thick filaments.
































































